Client’s Good Business Partner
Striving to contribute to the elimination of “drug lag”.
To help accelerate drug development, in addition to leveraging on Heishinkai’s strengths and project management expertise, trusting relationship building with clients and great effort on committing to even short start-up study timelines are strived for at all times.
Heishinkai Strength – Strong Sense of Knowing How to Enable Early Drug Development
Heishinkai’s strong sense of knowing how to enable early drug development assures our ability to offer high speed enrollment throughout the course of the study period, resulting in optimal overall conduct speed.
(1) Rapid Study Start-Up
Next-Day contracting post IRB review is possible. Upon request, expedited start-up is also possible via shortening key milestones lead time, e.g., contracting to SIV , IRB to FPFV.
(2) Quick Enrollment and High Average Enrollment Index at Over 50%
Upon SIV, our industry experienced team provides the study schedule created to offer the earliest possible start and shortest enrollment period. Throughout enrollment, the team also pays close attention and adjust per beneficial, resulting in the earliest recruitment completion possible.
Attention is also paid on the enrollment volume as well as enrollment oversight across all study sites and incoming client requests throughout recruitment. For example, even when the target number is achieved at one site, for the sake of other sites, enrollment continues at the same high speed.
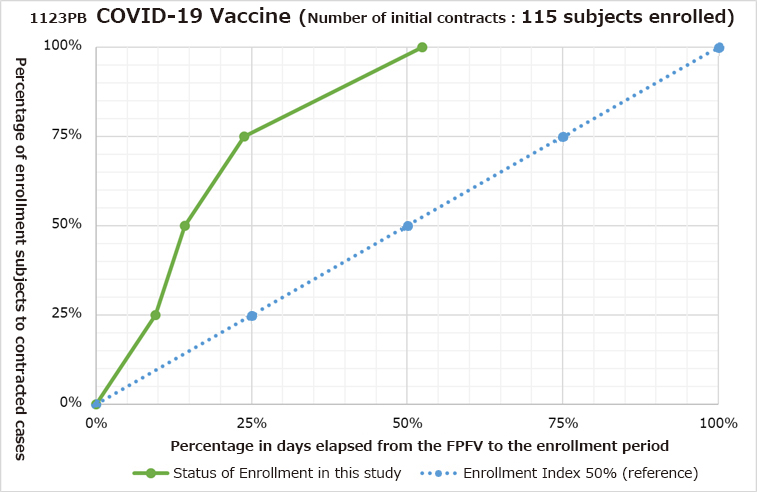
An enrollment index* is utilized as a unique evaluation index to measure subject enrollment speed. We have been achieving a high enrollment index of over 50%.
What is Enrollment Index?
Enrollment index is our useful tracking tool used to monitor enrollment progress and enhance recruiting speed. Actual recruitment time can be compared against the forecast achievement at any percentage. Illustrated in the figure below, the solid green line represents the actual achieved percentage in relation to time and the dotted blue line represents the forecast. It is clearly seen that at the forecast 50%, the actual achievement exceeds well above 50%.
Example – Enrollment Index of 76% in a clinical trial conducted in OCROM Clinic
Types of Clinical Trials
Heishinkai is capable of conducting a wide variety of studies including FIH (First in Human studies). We also aim to actively participate in clinical trials requiring uncommon trial procedures, uncommon testing equipment, and/or uncommon subject populations.
• First in Human studies
• First in Japanese studies
• Intensive QT studies
• Microdose studies
• DDI studies
• Skin irritation studies (patch test/photo patch test)
Special Clinical Pharmacology studies:
Bronchoalveolar lavage studies (BAL)
Intraocular pressure diurnal variation studies
Continuous glucose monitoring studies (CGM)
Studies in Special Populations:
Clinical pharmacology studies in patients
Clinical pharmacology studies in non-Japanese subjects
Studies in pediatric/elderly subjects
Studies using Special Tests:
Polysomnography (PSG)
Colonoscopy
ECHO
Pulmonary function
Electroencephalography (EEG)
Skin biopsy
Studies by Investigational Drug:
Vaccines
Narcotics
Biopharmaceuticals
Biosimilars
Antibodies
Other Trials
Investigator-Initiated clinical trials
Clinical research to harvest bioresources
